ni3 molecular geometry|molecular geometry vsepr theory : Bacolod In this tutorial, we will study NI3 lewis structure, molecular geometry, bond angle, polarity, hybridization, etc. Properties of Nitrogen triiodide. It appears as purple gas. It is not soluble in water but soluble . Check out this new search engine called Xyggy Legal. It went live on November 16, 2009. Xyggy offers more than just general legal searches, so you can reach Xyggy Patent, which covers United States patents from 1976 to present. Xyggy Articles searches through archived news articles of the New York Times from 1987 – 2007.
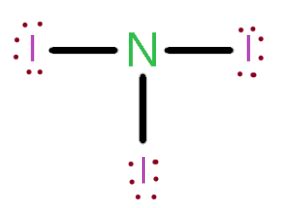
ni3 molecular geometry,An explanation of the molecular geometry for the NI3 (Nitrogen triiodide) including a description of the NI3 bond angles. The electron geometry for the Nitrogen triiodide is also.
In this tutorial, we will study NI3 lewis structure, molecular geometry, bond angle, polarity, hybridization, etc. Properties of Nitrogen triiodide. It appears as purple gas. It is not soluble in water but soluble .
3. 1K views 1 year ago Lewis Structure. NI3 is a chemical formula for Nitrogen Triiodide. This molecule consists of one Nitrogen and three Iodine atoms. To determine its Lewis Structure, we.ni3 molecular geometry molecular geometry vsepr theory A step-by-step explanation of how to draw the NI3 Lewis Dot Structure (Nitrogen Triiodide).For the NI3 structure use the periodic table to find the total num. Watch on. 6 Steps to Draw the Lewis Structure of NI3. Step #1: Calculate the total number of valence electrons. Here, the given molecule is NI3 (nitrogen .
D With two nuclei around the central atom and one lone pair of electrons, the molecular geometry of SnCl 2 is bent, like SO 2, but with a Cl–Sn–Cl bond angle of 95°. The molecular geometry can be described as a .NI 3 is highly explosive when dry. When it explodes it produces a cloud of purple smoke (this is iodine gas). It is termed a "contact explosive" since it doesn't take much to set it .

Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the . Steps. Use these steps to correctly draw the NI 3 Lewis structure: #1 First draw a rough sketch. #2 Mark lone pairs on the atoms. #3 Calculate and mark formal charges on the atoms, if required. Let’s .Draw the Lewis structure for CCl2F2 and provide the following information. a. molecular geometry b. electron geometry c. hybridization of the central atom d. polarity; Draw the Lewis structure and write the molecular geometry and hybridization on the central atom, and polar or nonpolar for SeO_2.Use VSEPR theory to predict the molecular geometry of the molecule HI: 1. tetrahedral 2. trigonal-bipyramidal 3. trigonal-planar 4. trigonal-pyramidal 5. bent or angular 6. linear 7. octahedral 8. None of these; The molecular geometry of the NF3 is a) trigonal pyramidal b) trigonal planar c) bent d) tetrahedral e) T-shaped
I3- is an interesting and difficult molecule to deal with when it comes to chemical bonding. Although the molecular geometry is linear as discussed earlier, the electronic geometry is trigonal bipyramidal. The . The total valence electron is available for drawing the BI3 Lewis structure is 24. The molecular geometry or shape of BI3 is trigonal planar. BI3 is nonpolar and has Sp 2 hybridization. In the BI3 Lewis structure, a total of 18 nonbonding electrons and 6 bonded electrons are present.
Geometry of Molecules. Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. Lewis Structure is the name given to such a skeletal diagram where we use the symbols of the atoms and use dots to represent the valence shell electrons. Hence, Lewis Structure is also commonly called Electron Dot Structure. Let us proceed to draw the most appropriate LS diagram of CO32- ion. Step 1: Count the Total Number of Valence .
molecular geometry vsepr theory The molecular geometry of Nitrogen Triiodide (NI3) based on the VSEPR model is trigonal pyramidal, due to presence of three single bonds and one lone pair of electrons on the central nitrogen atom. Explanation: Using the VSEPR model, nitrogen triiodide (NI3) has a trigonal pyramidal molecular geometry. This is based on the .
NF3 Molecular Geometry. Molecular geometry or molecular shape is an important concept that we need to decipher while we are learning the chemical bonding of any chemical composition. While Lewis Structure gives us an idea about the internal bond types and valence electron sharing inside a given molecule, it can only explain a two . DO NOT FORGET TO SUBSCRIBE!LinkedIn: https://www.linkedin.com/in/kevan-j-e.Snapchat: https://www.snapchat.com/add/kravonoInstagram: .

A bond distance (or bond length) is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Bond distances are measured in Ångstroms (1 Å = 10 –10 m) or picometers (1 pm = 10 –12 m, 100 pm = 1 Å). Figure \boldsymbol10.3.1 \boldsymbol 10.3. 1: Bond distances (lengths) and angles are shown for the .
ni3 molecular geometryA bond distance (or bond length) is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Bond distances are measured in Ångstroms (1 Å = 10 –10 m) or picometers (1 pm = 10 –12 m, 100 pm = 1 Å). Figure \boldsymbol10.3.1 \boldsymbol 10.3. 1: Bond distances (lengths) and angles are shown for the .
It's a covalent molecule, which means the atoms in the molecule share electrons. Nitrogen triiodide is a very reactive substance that can explode with just a slight vibration and typically leave orange-to-purple stains in the wake of an explosion. It's usually used as an example of highly-sensitive chemicals to high school chemistry students.
There are three single bonds and one lone pair of electrons in the NH3 molecule. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. The shape is . Due to its polymeric nature, the hybridization of SeO2 is sp3. SeO2 has a bent molecular structure with bond angles of 120°. Selenium Dioxide is a unique polymeric compound. Check this article on SeO2 to .Question: Determine the molecular geometry of NI3. Identify the bond angle in NI3. Determine the molecular geometry of NI3. Identify the bond angle in NI3. Show transcribed image text. There’s just one step to solve this. Who are the experts? Experts have been vetted by Chegg as specialists in this subject.Figure 10.2.2 ): (CC BY-NC-SA; anonymous) The two oxygens are double bonded to the sulfur. The oxygens have 2 lone pairs while sulfur had one lone pair. 3. There are two bonding pairs and one lone pair, so the structure is designated as AX 2 E. This designation has a total of three electron pairs, two X and one E.Question: What is the electron and molecular geometry of NI3?Question options:trigonal planar, trigonal planartetrahedral, tetrahedraltetrahedral, trigonal planartetrahedral, trigonal pyramidaltetrahedral, bent. What is the electron and molecular geometry of NI3? There are 2 steps to solve this one. Contributors and Attributions. John S. Hutchinson (Rice University; Chemistry) 7: Molecular Geometry and Electron Domain Theory is shared under a license and was authored, remixed, and/or curated by LibreTexts. We begin by assuming a Lewis structure model for chemical bonding based on valence shell electron pair sharing and .Question: Draw the Lewis dot structure for NI3. Determine the electron geometry of NL3. trigonal planar linear tetrahedral Determine the molecular geometry of NI3. Identify the bond angle in NI3. \begin {tabular} {ll} bent & 180∘ \\ \hline tetrahedral & 109.5∘ \\ \hline \end {tabular} There are 2 steps to solve this one.
ni3 molecular geometry|molecular geometry vsepr theory
PH0 · ni ground state electron configuration
PH1 · molecular geometry vsepr theory
PH2 · molecular geometry practice quiz
PH3 · molecular geometry list
PH4 · molecular geometry chart
PH5 · how to determine molecular geometry
PH6 · electron and molecular geometry table
PH7 · ch3 electron pair geometry
PH8 · Iba pa